CMS recently published the FY 2023 Inpatient Prospective Payment System (IPPS) Final Rule which includes several changes to the Hospital Inpatient Quality Reporting (IQR) Program and Medicare Promoting Interoperability Program for eligible hospitals and critical access hospitals (CAHs). For the full text of the rule, see the Federal Register.
Hospital Inpatient Quality Reporting (IQR) Program
CMS finalized numerous changes related to the Hospital Inpatient Quality Reporting (IQR) Program including the adoption of ten new measures and the refinement of two existing measures.
New Measures
In this IPPS final rule, CMS has finalized ten new measures with voluntary and mandatory reporting periods beginning with the CY 2023 reporting year. The table below summarizes the newly adopted measures including the measure type and reporting timeline.
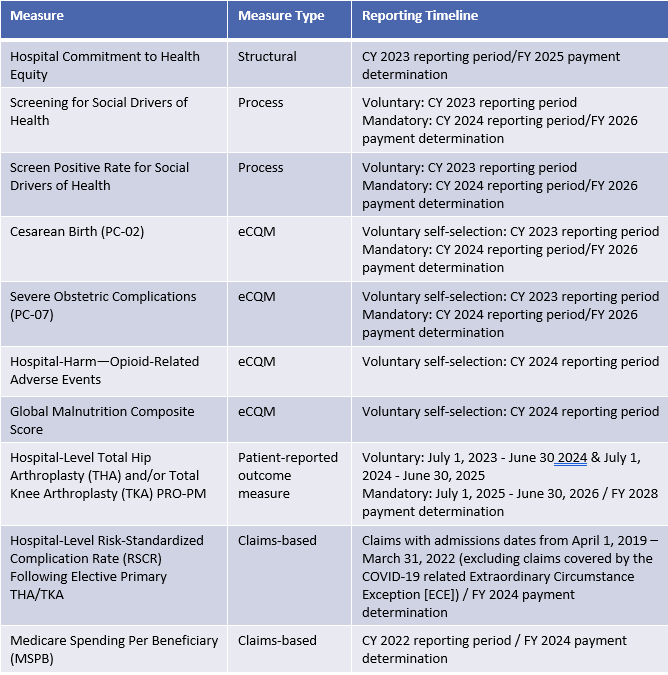
Measure Modifications
CMS finalized refinements to two measures beginning with the FY 2024 payment determination as follows:
- Hospital‐Level, Risk‐Standardized Payment Associated with an Episode-of-Care for Primary Elective THA and/or TKA
- Expand the measure outcome to include 26 clinically vetted mechanism complication ICD-10 codes
- Excess Days in Acute Care (EDAC) After Hospitalization for Acute Myocardial Infarction (AMI)
- Increase the minimum case count of 25 to a minimum case count of 50 during the measurement period
Other IQR Changes
CMS finalized establishing a hospital quality designation to capture the quality and safety
of maternity care that will be publicly reported on a CMS website beginning fall of 2023. Hospitals will receive this designation based on their attestation to the Maternal Morbidity Structural Measure.
Beginning with the CY 2024 reporting period / FY 2026 payment determination, CMS finalized increasing the eCQM reporting requirements from four eCQMs (one mandatory and three self-selected) to six eCQMs (three mandatory and three self-selected).
CMS finalized modifying the eCQM validation policy beginning with CY 2022 data (affecting FY 2025 payment determination) by increasing the submission requirement from 75% to 100% of the requested medical records to successfully complete eCQM validation. The accuracy of eCQM data submitted for validation does not affect the validation score.
Beginning with the FY 2026 payment determination, CMS finalized the removal of zero denominator declarations and case threshold exemptions policies for hybrid measures.
Medicare Promoting Interoperability (PI) Program
CMS has finalized several changes to the Medicare Promoting Interoperability (PI) Program related to the objective measures and scoring beginning with the CY 2023 EHR Reporting Period. The following changes were finalized for CY 2023 EHR reporting period unless otherwise noted.
- Require Query of Prescription Drug Monitoring Program (PDMP) and expand to include Schedule II, III, and IV drugs and maintain the associated points at 10 points
- Add a new yes/no attestation measure, Enabling Exchange under the Trusted Exchange Framework and Common Agreement (TEFCA) measure under the Health Information Exchange (HIE) Objective, which would be an optional alternative to the three existing measures under the HIE Objective
- Add an Antimicrobial Use and Resistance (AUR) Surveillance measure requiring its reporting under the Public Health and Clinical Data Exchange Objective beginning with the EHR reporting period in CY 2024.
- Reduce the active engagement options for the Public Health and Clinical Data Exchange Objective from three to two options: Option 1: Pre-production and Validation and Option 2: Validated Data Production
- Require submission of the level of active engagement, in addition to submitting the measures for the Public Health and Clinical Data Exchange Objective. Beginning with CY 2024 EHR reporting period, hospitals may only spend one EHR reporting period at Option 1 before progressing to Option 2 for a particular measure.
- Establish public reporting of certain Medicare Promoting Interoperability Program data
- Changes to the PI scoring methodology
- Increase the Public Health and Clinical Data Exchange Objective from 10 to 25 points
- Increase the points for the Electronic Prescribing Objective from 10 to 20
- Reduce the points for the Health Information Exchange Objective from the current 40 points to 30 points
- Reduce the points for the Provide Patients Electronic Access to Their Health Information from the current 40 to 25 points
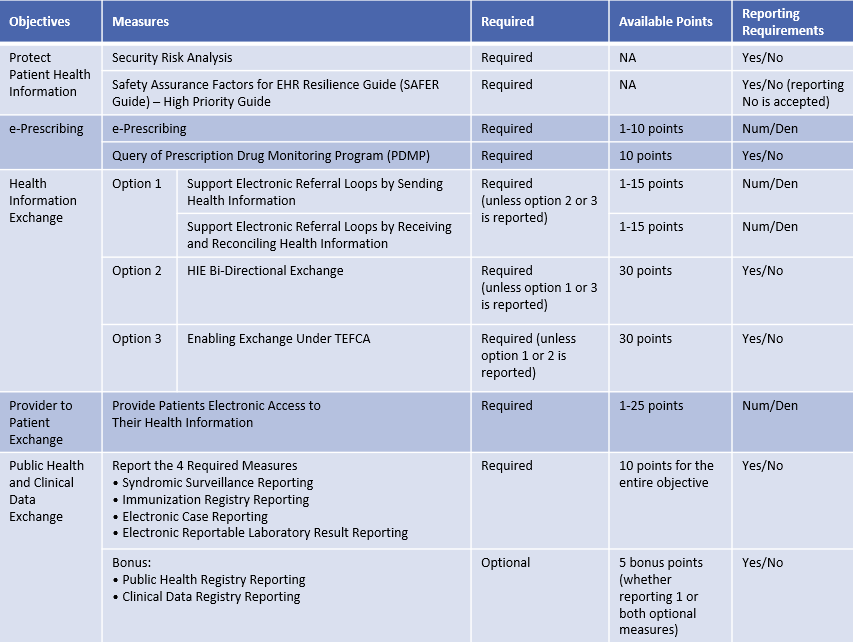
Source: CMS IPPS FY2023 Final Rule
CMS finalized changes to eCQM reporting for PI in alignment with that of IQR. CMS finalized the adoption of two new eCQMs beginning with CY 2023 reporting (PC-02 and PC-07), and two new eCQMs beginning with CY 2024 reporting (Hospital Harm Opioid Related Adverse Events and Global Malnutrition Composite Score). The self-selection and mandatory reporting timelines align with that of IQR. CMS also finalized modifying the eCQM reporting and submission requirements to increase eCQM reporting from four eCQMs (one mandatory and three self-selected) to six eCQMs (three mandatory and three self-selected) beginning with the CY 2024 reporting period in line with the IQR program.
If you have questions about your hospital reporting of eCQMs, Promoting Interoperability measures, or chart-abstracted measures, please contact us.

