CMS recently released the FY 2020 Inpatient Prospective Payment Systems (IPPS) Final Rule which continues to focus on transforming the healthcare delivery system to provide patients with better care. To this end, CMS has finalized changes to the Hospital Inpatient Quality Reporting (IQR) program and Medicare & Medicaid Promoting Interoperability (PI) program for eligible hospitals and critical access hospitals (CAHs). (For the full text of the final rule, see the Federal Register.)
Hospital Inpatient Quality Reporting (IQR) Program
For the Hospital Inpatient Quality Reporting (IQR) Program, CMS finalized some changes to the set of measures. Although CMS proposed adopting two new opioid-related electronic clinical quality measures (eCQMs) beginning with the CY 2021 reporting period (FY2023 payment determination), CMS only finalized Safe Use of Opioids – Concurrent Prescribing. CMS did not finalize Hospital Harm – Opioid-Related Adverse Events. Beginning with the July 1, 2023 through June 30, 2024 reporting period for the FY 2026 payment determination, CMS finalized the removal of the Claims-Based Hospital Wide All-Cause Readmission measure and replaced it with the Hybrid Hospital-Wide-All-Cause Readmission (Hybrid HWR) Measure which includes both claims and electronic health record (EHR) data. Prior to this, CMS finalized voluntary reporting periods for the Hybrid HWR measure which run from July 1, 2021 through June 30, 2022 and July 1, 2022 through June 30, 2023. These voluntary reporting periods would not impact payment nor be publicly reported, but the hospital will receive a confidential hospital-specific feedback report. After the end of the voluntary reporting periods, CMS finalized the Hybrid HWR measure results would be publicly reported beginning with the data collected in CY 2023 impacting FY 2026 payment determination.
The rule also finalized its proposals regarding the eCQM reporting requirements for upcoming years. For the CY 2020 reporting period (FY 2022 payment determination) and CY 2021 reporting period (FY2023 payment determination), CMS finalized that hospitals will continue to submit one, self-selected calendar quarter of data for four self-selected eCQMs in the Hospital IQR Program measure set. However, for the CY 2022 reporting period (FY2024 payment determination), CMS finalized that hospitals will be required to report one, self-selected calendar quarter of data for four eCQMs where three are self-selected eCQMs and one is the finalized Safe Use of Opioids – Concurrent Prescribing eCQM. EHR Technology must continue to be certified to all eCQMs available to report for the CY 2020 reporting period and subsequent years.
Medicare & Medicaid Promoting Interoperability Programs
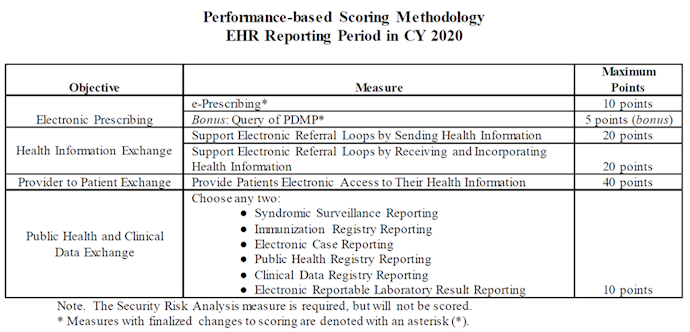
CMS finalized changes to the Medicare and Medicaid Promoting Interoperability (PI) Programs with the aim to reduce administrative burden, continue the use of 2015 Edition CEHRT, and improve patient access to their EHRs so they can make fully informed health care decisions.
CMS finalized that new and returning hospitals participating in the Medicare Promoting Interoperability (PI) Program will report to CMS a minimum of any continuous 90-day period in CY 2021. CMS also finalized beginning with CY 2020 that hospitals under the Medicare PI Program would attest to numerators and denominators of PI measures that include only actions that have occurred during the EHR reporting period selected by the hospital. The one exception to this is the Security Risk Analysis measure, which may occur any time during the calendar year in which the EHR reporting period occurs.
CMS finalized changing Query of PDMP measure from a required to an optional measure and available for 5 bonus points for CY2020. CMS thus removed the CY 2020 exclusion for this measure as it would be optional to report. Furthermore, beginning with CY 2019 reporting, CMS finalized converting this measure from a numerator/denominator to a yes/no attestation, in which a “yes” response would indicate that for at least one Schedule II opioid electronically prescribed using CEHRT during the reporting period, the hospital used data from CEHRT to conduct a query of a PDMP for prescription drug history. Beginning with the CY 2020 reporting period, CMS finalized the removal of the Verify Opioid Agreement measure from the PI Program. This measure remains a numerator/denominator optional measure available for 5 bonus points in CY2019.
CMS plans to continue to align the CQM reporting requirements for the PI Programs with those for the Hospital IQR Program. Although CMS proposed adopting two new opioid-related electronic clinical quality measures (eCQMs) beginning with the CY 2021 reporting period (FY2023 payment determination), CMS only finalized Safe Use of Opioids – Concurrent Prescribing. CMS did not finalize Hospital Harm – Opioid-Related Adverse Events.
To reduce burden for hospitals, CMS finalized its proposal to align the reporting period requirements for PI with IQR. To this end, hospitals will continue to submit one, self-selected calendar quarter of data for four self-selected eCQMs in the PI Program measure set for the CY 2020 reporting period and CY 2021 reporting period. However, for the CY 2022 reporting period (FY2024 payment determination), hospitals will be required to report one, self-selected calendar quarter of data for four eCQMs where three are self-selected eCQMs and one is the finalized Safe Use of Opioids – Concurrent Prescribing eCQM. Hospitals that must submit to CMS by attestation where electronic reporting is not feasible will have to report on all CQMs in the PI measure set for the full calendar year (consisting of 4 quarterly data reporting periods). Though, beginning with CY 2023 reporting, CMS finalized its proposal to eliminate attestation as a method for reporting CQMS for Medicare PI Program and instead require all hospitals to submit their CQM data electronically. EHR Technology must continue to be certified to all eCQMs available to report for the CY 2020 reporting period and subsequent years.
Should you have questions about your hospital reporting of eCQMs, Promoting Interoperability measures, or chart-abstracted measures, please contact us.

